Experimental & Translational Psychiatry
Leader: Dr. Alexandre Surget
Co-Leader: Pr. Wissam El Hage
Co-Leader: Pr. Wissam El Hage
Our team's project is centered around three strategies:
- To promote basic/clinical research that relies on multi-scale examinations of brain dysfunctions and psychiatric disorders.
- To foster bidirectional translation between bench and bedside.
- To favor precision psychiatry and approaches relying on biologically-based constructs. (RDoC, NIMH 2009)
Objectives
- To revealing the neurobiological and neurofunctional basis of altered cognitive and affective dimensions in psychiatric disorders, focusing on disorders affecting the mood, stress and reward systems.
- To identify signatures of vulnerability/resilience, for diagnosis and for treatment response/resistance.
- To develop innovative, efficient preventive and therapeutic approaches in psychiatry.
Specific research axes
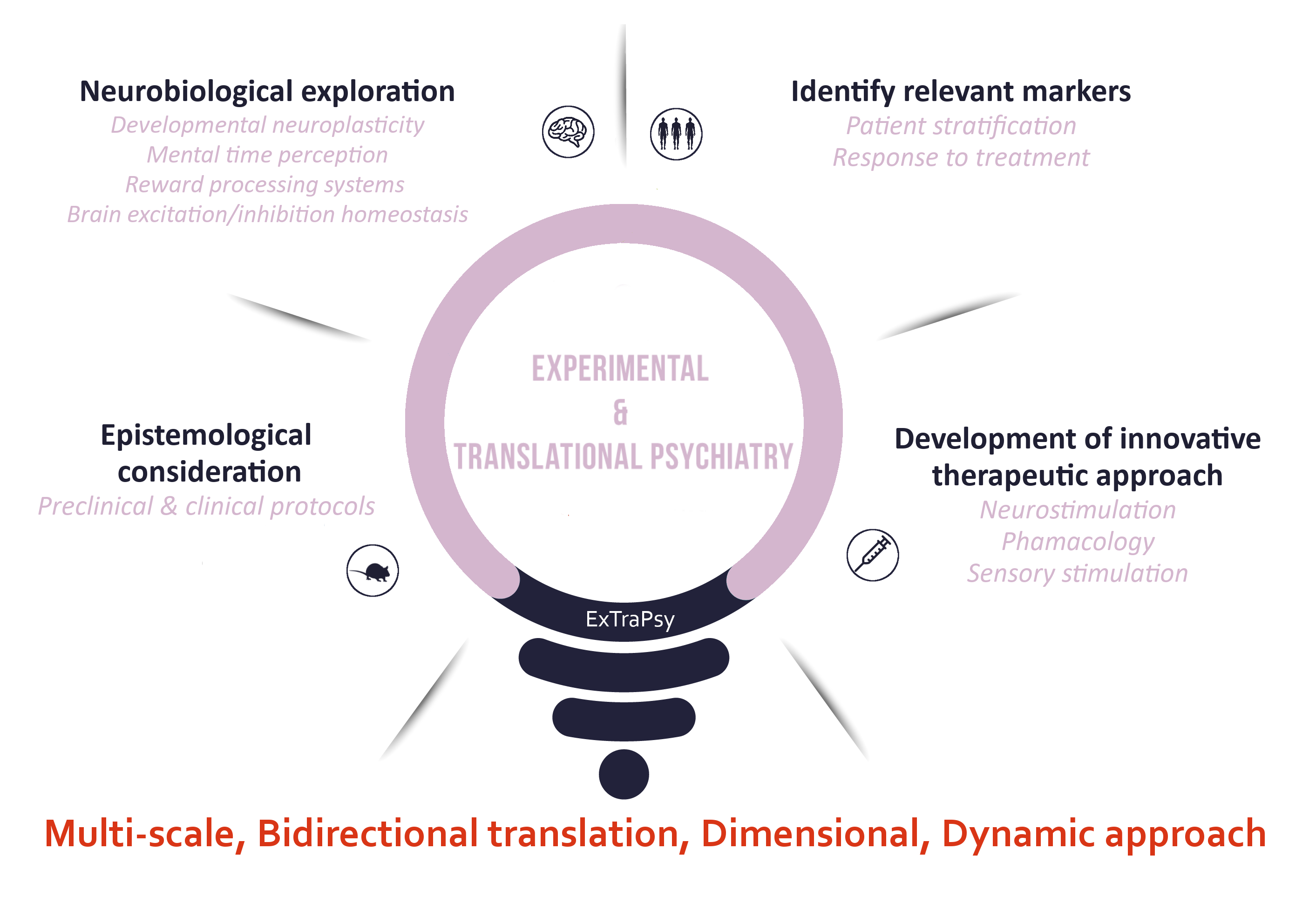
Neurobiological exploration
This axis aims to uncover the neurobiological mechanisms underlying stress-, mood-, cognitive- and reward- processing dysfunctions in different psychiatric disorders.This axis includes the study of:
- Developmental neuroplasticity and how they are impacted by early-life adversity (ELA).
- Time perception and encoding, its neural basis and alterations in stress-related disorders (depression, PTSD).
- Adult neurogenesis in depression and PTSD.
- Reward-processing systems in mouse models of ASD.
- Brain excitation/inhibition homeostasis.
- Psycho-socio-biological correlates of addictive disorders.
Identification of relevant signatures for patient stratification, personalized prevention and therapy
This axis aims to identify relevant markers for patient stratification, from vulnerability and diagnosis to response to preventive and therapeutic interventions.In particular, we wish to determine if neurobiological determinants qualify as candidate biomarkers in validated animal models and in patients. We also investigate the prognostic value of neurosensory (olfactory perception), neurovascular (cerebral pulsatility via ultrasound imaging), neuroanatomical changes (MRI, radiotracers for PETscans) for response to treatment. We strive to identify functional individual profiles in addictive disorders looking at the contribution of psychiatric comorbidities (especially ADHD) and using radiopharmaceutical multitracer approaches.
Development of innovative therapeutic approaches
Within this aim, we aim to characterize the neurobiological effects and therapeutic efficacy of:- Innovative neurostimulation including repetitive transcranial magnetic stimulation (rTMS), transcranial focal ultrasound stimulation (tFUS), transcranial direct current stimulation (tDCS).
- Pharmacological approaches (e.g. nitric oxide and nanobodies).
- Sensory stimulations.
Epistemological consideration
We study the epistemological reasons for using preclinical and clinical protocols looking at the history of animal models in biological psychiatry and the epistemological and ethical issues they raise.
Functional Groups
Exploration of Stress and mood disorders in preclinical models
Resp. Dr. Alexandre Surget
Objectives:
The Team will use preclinical models of stress, depression and PTSD in mice at different stages of development and lifespan (from juvenile to older adults),  Several projects are currently underway:
Several projects are currently underway:
Objectives:
The Team will use preclinical models of stress, depression and PTSD in mice at different stages of development and lifespan (from juvenile to older adults),
- To investigate the neurobiological underpinnings of stress-related dysfunctions
- To identify biological, neurobehavioral and cognitive signatures linked to models of stress, depression and stress-related disorders
- To test innovative preventive or therapeutic strategies in these models

- Neurobiological underpinnings of earlylife adversity (ELA)
- Alterations of time perception and encoding in stressrelated disorders as a cognitive signature and their neural bases in mouse models
- Impact of innovative neurostimulation strategies in depression and PTSD
- Role of adult hippocampal neurogenesis in pathophysiology, prevention and treatment of depression and PTSD
Clinical research on stress, mood and reward systems dysfunctions
Resp. Pr. Wissam El-Hage
Objectives:
Developments and characterization of therapeutic efficacy of: 
Several projects are currently underway:
Objectives:
Developments and characterization of therapeutic efficacy of:
- Innovative neurostimulation including repetitive transcranial magnetic stimulation (rTMS), transcranial focal ultrasound stimulation (tFUS), transcranial direct current stimulation (tDCS)
- Pharmacological approaches (e.g. nitric oxide)
- Sensory stimulations, in patients with PTSD, MD, behavioral addictions as well as in treatment-resistant MD patients

Several projects are currently underway:
- Olfactory perception and stimulation in depression
- Effects of nitrous oxide (N2O) as a potential fastacting antidepressant
- Novel therapeutic brain stimulation strategies to be tested in homogeneous subgroups of patients and/or targeted biomarkers
- Effectiveness of therapeutic interventions in different types of behavioral addictions
- Association of addictive disorders with ADHD
Exploration of Social Anhedonia in preclinical models
Resp. Dr. Julie Lemerrer and Dr. Jérôme Becker
Objectives: 
Several projects are currently underway:
Objectives:
Our group investigates neurodevelopmental disorders in preclinical models, with a focus on Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD). We combine mechanistic in vitro approaches, allowing to nominate therapeutic targets and pathways, with in vivo pharmacology to establish causal links and assess therapeutic potential of innovative approaches. In parallel, multi-omics analyses define molecular signatures associated with behavioral phenotypes.
We aim to:
- Identify the neurobiological underpinnings of social anhedonia and withdrawal under physiological and pathological conditions such as ASD, with a primary focus on the role of striatal Medium Spiny Neurons (MSN) in controlling social reward processes,
- Evaluate the impact of early exposure to environmental pollutants (fungicides, micro- and nano-plastics), bacterial toxins (p-Cresol) or western diet on the susceptibility to develop neurodevelopmental disorders,
- Explore orphan G protein–coupled receptors (GPCRs) expressed in the striatum as potential therapeutic targets in neurodevelopmental and psychiatric disorders, and decode the related molecular signaling pathways,
- Develop novel therapeutic strategies to relieve key behavioral impairments relevant to neurodevelopmental disorders across multiple mouse models, specifically by identifying, characterizing and engineering single domain antibodies, so called “nanobodies”, targeting GPCRs.

Several projects are currently underway:
- A2utismAb (ANR 2021-2026) explores the potential of nanobodies targeting the adenosine A2A receptor to relieve ASD-like symptoms,
- FunExpo (ANR 2023-2026) evaluates the effects of early exposure to anilinopyrimidine fungicides on brain development and susceptibility to neurodevelopmental (ASD) and neurodegenerative (Alzheimer’s disease) disorders,
- OT-ism (ANR 2025-2027) aims to develop novel non-peptide agonists of the oxytocin receptor for therapeutic use in ASD,
- EXPOSIGNALZ (Horizon Health 2025-2030) investigates the effects of chemical pollutants on the development of neurodegenerative diseases, notably Alzheimer's disease, and how early exposure can prime susceptibility to neurodegeneration,
- Nano4Autism (ANR 2025-2029) proposes to identify nanobodies targeting the glutamate receptor mGlu4, within homo- or heterodimers, as innovative therapeutic tools for ASD.
- MT2-ASD (ERDERA 2026-2029) focuses on advancing a selective melatonin MT2 receptor agonist toward a targeted therapy for ASD.
French presentation
Présence d'un lecteur vidéo
Fundings




Industrial partners


Medias
Présence d'un lecteur vidéo
Présence d'un lecteur vidéo
Présence d'un lecteur vidéo
Présence d'un lecteur vidéo
Présence d'un lecteur vidéo