Therapeutic ultrasound
1. PHYSICS OF UCA/ULTRASOUND INTERACTION
The aim of this research is, first, to develop theoretical models to simulate the behavior of microbubble ultrasound contrast agents (UCA) in experimental and clinical conditions, and second, to carry out the experimental validation of these models. Medical applications that mainly determine the scope of our research include targeted ultrasound imaging and targeted drug delivery by sonoporation. In the context of these
applications, it is planned to develop models for the following problems: the effect of boundaries, such as walls of blood vessels and experimental containers, on the motion and the acoustic response of UCA, acoustic microstreaming produced by UCA near boundaries, stresses exerted by this microstreaming on neighboring objects such as cells and tissues, the effect of the rheological properties of the encapsulating shell on the dynamics of UCA, collective effects in UCA populations. The study of the above-mentioned problems is expected to make it possible to reveal, in particular, which changes caused by boundaries in the acoustic response of UCA can be used to distinguish the scattered echoes produced by UCA attached to a blood vessel wall from echoes produced by freely circulating non-adherent agents. The other purpose of our work is to establish physical mechanisms responsible for cell membrane permeabilization caused by sonoporation.
Investigators: A.A. Doinikov, C. Sennoga
Project leader: A. Bouakaz
Collaborations: Bracco Research, Suisse
applications, it is planned to develop models for the following problems: the effect of boundaries, such as walls of blood vessels and experimental containers, on the motion and the acoustic response of UCA, acoustic microstreaming produced by UCA near boundaries, stresses exerted by this microstreaming on neighboring objects such as cells and tissues, the effect of the rheological properties of the encapsulating shell on the dynamics of UCA, collective effects in UCA populations. The study of the above-mentioned problems is expected to make it possible to reveal, in particular, which changes caused by boundaries in the acoustic response of UCA can be used to distinguish the scattered echoes produced by UCA attached to a blood vessel wall from echoes produced by freely circulating non-adherent agents. The other purpose of our work is to establish physical mechanisms responsible for cell membrane permeabilization caused by sonoporation.
Investigators: A.A. Doinikov, C. Sennoga
Project leader: A. Bouakaz
Collaborations: Bracco Research, Suisse
 In many mammalian species, vascular development of the placenta represents a crucial process for adequate fetal growth and development. During human pregnancy, normal placental development results in remodeling the maternal spiral arteries to allow increased delivery of maternal blood through a low-pressure, low-velocity placental bed. Several pregnancy complications such as preeclampsia, placental abruption,
In many mammalian species, vascular development of the placenta represents a crucial process for adequate fetal growth and development. During human pregnancy, normal placental development results in remodeling the maternal spiral arteries to allow increased delivery of maternal blood through a low-pressure, low-velocity placental bed. Several pregnancy complications such as preeclampsia, placental abruption,intrauterine growth restriction or even preterm labor have been related to vascular dysfunction in the placental bed and are major causes of maternal and fetal morbidity and mortality. Conventional B-mode US or uterine artery Doppler, remained imperfect tools to evaluate maternal blood perfusion to the placenta. CEUS could offer new perspectives in screening or early prediction of obstetrical complications related to impaired placentation.
CEUS also enable quantitative analysis of placental perfusion to better evaluate the different aspects and degrees of placental insufficiency. Moreover VEGF-labeled microbubbles are an additional tool to elucidate the adaptatives mechanisms during placental insufficiency. Our objective is to develop a quantitative tool for small animal placental insufficiency models and to evaluate the impact of various therapeutic agents on utero-placental perfusion using CEUS. The use of VEGF-targeted microbubble and targeted drug delivery (sonoporation) directly inside the intervillous space of the placenta may offer new therapeutics modalities.
Investigators: C. Fouché-Arthuis
Project leader: F. Perrotin
Collaborations: F. Tranquart (Bracco Research, Suisse)
Début de la page
3. SONOPORATION FOR TARGETED DRUG AND GENE DELIVERY
Début de la page
Investigators: A. Zeghimi, J-M. Escoffre
Project leader: A. Bouakaz
Collaborations: F. Tranquart (Bracco Research, Switzerland), R. Uzbekov (PPF, Dept. Microscopies, Tours), K. Kostarelos (UCL-SOP, UK)
Project leader: A. Bouakaz
Collaborations: F. Tranquart (Bracco Research, Switzerland), R. Uzbekov (PPF, Dept. Microscopies, Tours), K. Kostarelos (UCL-SOP, UK)
Début de la page
Plasmid DNA (pDNA) is a highly attractive molecule for gene therapy, provided that efficient, safe, and targeted delivery can be achieved. Recent investigations have shown that the application of low-frequency ultrasound (1 to 10 MHz) in combination with contrast agents enhances the intracellular delivery of genes. The ultrasound-induced oscillations of microbubbles are assumed to play a major role in the increased uptake of
genes by cells (also called as sonoporation). Because ultrasound exposure can be controlled spatially and temporally, microbubble-assisted ultrasound can provide an advantageous and safe targeted delivery method for
in-vivo applications. However, the currently available literature indicates that the potential of this method for gene transfection could be improved further. This optimization could be achieved by controlled and targeted gene delivery and by finding ways to deliver a greater amount of genes into the targeted tissues. Indeed, independently of the acoustic (e.g., pressure, insonation time, etc.) and biological (e.g., pDNA concentration, cell
lines, etc.) parameters, the highest ultrasound- and microbubble-mediated transfection levels achieved in-vitro did not exceed 50%. The main objectives of this research project are the understanding of mechanisms involved in the gene delivery using microbubble-assisted ultrasound and its optimization for its translation in clinics.
Using glioblastoma cells, an unprecedented transfection rate of 70% is reached with Vevo Micromarker, corresponding to a 1.5-fold increase compared with the rate achieved with the other microbubbles. Moreover, attenuation and destruction were shown to be two key parameters in sonoporation efficiency.
Investigators: J-M Escoffre, G. Li, A. Novell, A. Zeghimi
Project leader: A. Bouakaz
Collaborations: F. Tranquart (Bracco Research, Switzerland), M-P Rols (IPBS-CNRS UMR 5089, Toulouse)
Début de la page
genes by cells (also called as sonoporation). Because ultrasound exposure can be controlled spatially and temporally, microbubble-assisted ultrasound can provide an advantageous and safe targeted delivery method for
in-vivo applications. However, the currently available literature indicates that the potential of this method for gene transfection could be improved further. This optimization could be achieved by controlled and targeted gene delivery and by finding ways to deliver a greater amount of genes into the targeted tissues. Indeed, independently of the acoustic (e.g., pressure, insonation time, etc.) and biological (e.g., pDNA concentration, cell
lines, etc.) parameters, the highest ultrasound- and microbubble-mediated transfection levels achieved in-vitro did not exceed 50%. The main objectives of this research project are the understanding of mechanisms involved in the gene delivery using microbubble-assisted ultrasound and its optimization for its translation in clinics.
Using glioblastoma cells, an unprecedented transfection rate of 70% is reached with Vevo Micromarker, corresponding to a 1.5-fold increase compared with the rate achieved with the other microbubbles. Moreover, attenuation and destruction were shown to be two key parameters in sonoporation efficiency.
Investigators: J-M Escoffre, G. Li, A. Novell, A. Zeghimi
Project leader: A. Bouakaz
Collaborations: F. Tranquart (Bracco Research, Switzerland), M-P Rols (IPBS-CNRS UMR 5089, Toulouse)
Début de la page
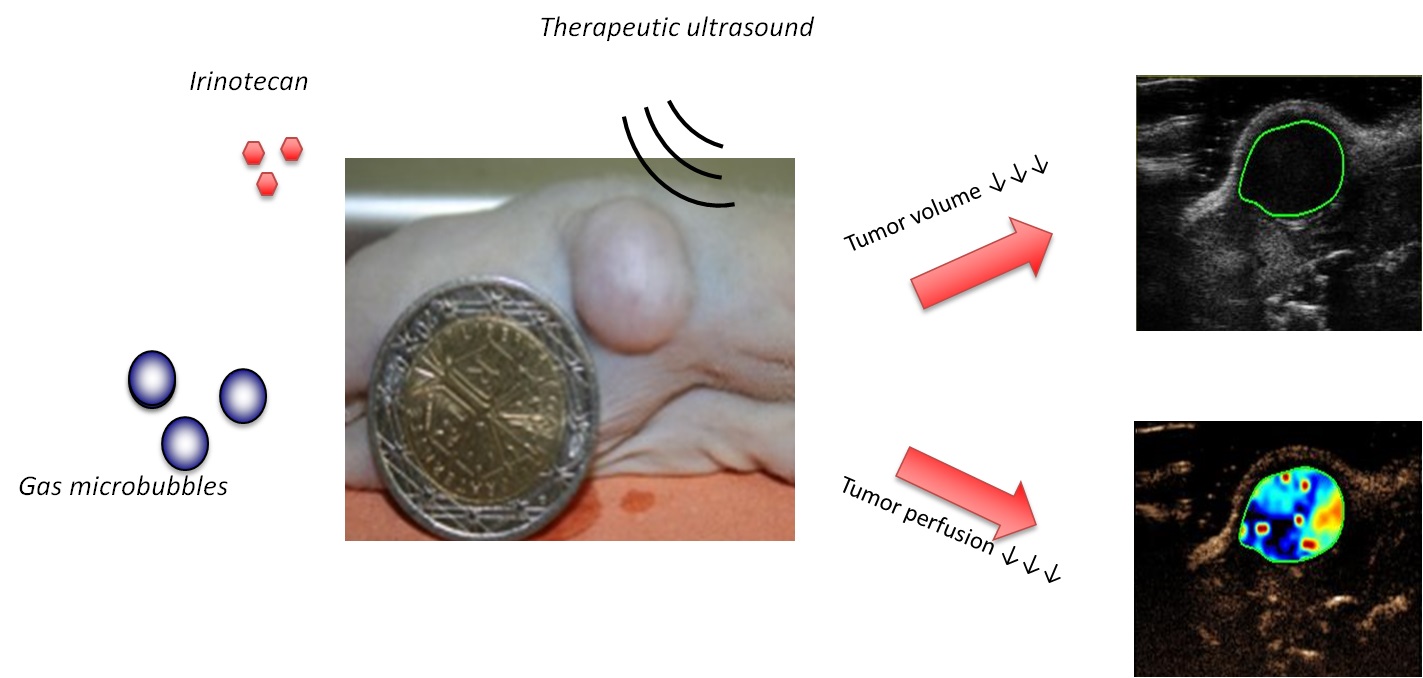 The early use of anticancer drugs (e.g., doxorubicin and irinotecan) in clinical application revealed major undesired effects, such as the development of resistance in tumor cells and side effects, such as the toxicity in healthy tissues (e.g., heart, brain, liver and kidney toxicities).To overcome these problems, the development of an efficient and targeted delivery of anticancer drugs is required to increase the local concentration at desired site while minimizing side effects to healthy tissues. As previously described, recent research has demonstrated that the application of high frequency ultrasound combined with ultrasound contrast agents enhances the extravasation and the intracellular delivery of drugs on cells and tissues.
The early use of anticancer drugs (e.g., doxorubicin and irinotecan) in clinical application revealed major undesired effects, such as the development of resistance in tumor cells and side effects, such as the toxicity in healthy tissues (e.g., heart, brain, liver and kidney toxicities).To overcome these problems, the development of an efficient and targeted delivery of anticancer drugs is required to increase the local concentration at desired site while minimizing side effects to healthy tissues. As previously described, recent research has demonstrated that the application of high frequency ultrasound combined with ultrasound contrast agents enhances the extravasation and the intracellular delivery of drugs on cells and tissues.In this context, we investigated the "coadministration approach" for the in-vitro delivery of doxorubicin and in-vivo delivery of irinotecan. This approach consists in the co-injection of gas microbubbles with free drug followed by the ultrasound application. Thus we reported that the doxorubicin delivery aided by microbubbleassisted ultrasound enhanced the death of breast cancer and glioblastoma cells, including the induction of apoptosis. Various microbubbles were evaluated including Vevo Micromarker, BR14, SonoVue and experimental polymer shelled microbubbles. The results showed that Vevo Micromarker microbubble-assisted ultrasound could induce an enhancement of doxorubicin in glioblastoma and breast cancer cell death. Moreover, we recently validated that the "coadministration approach" is efficient strategy for in-vitro and in-vivo irinotecan delivery in glioblastoma cells and tumors. Indeed, in-vitro results showed that the irinotecan treatment with microbubble-assisted ultrasound induced a significant decrease in cell viability of human glioblastoma cells. Then, using subcutaneous glioblastoma xenografts, the in-vivo preclinical study in nude mice demonstrated that this therapeutic protocol led to a decrease in tumor growth and perfusion and an increase of tumor necrosis.
The conclusions drawn from this study demonstrate the promising potential of this therapeutic approach for the anti-cancer targeted therapy.
Another approach to reduce side effects consists in the encapsulation of the drug into liposomes. For this purpose, our collaborators, Geers et al., designed doxorubicin liposomes-loaded microbubbles. Hence, the doxorubicin is encapsulated in liposomes that bind to the lipid shell of the microbubbles through a covalent link.
The clinical potential of the doxorubicin liposome-loaded microbubbles rests on ultrasound-triggered doxorubicin delivery monitored by ultrasound contrast imaging. We demonstrated that these microbubbles scatter sufficient signal for nonlinear ultrasound imaging and can thus be imaged in real time and be tracked in-vivo.
In-vitro therapeutic evaluation showed that ultrasound in combination with the doxorubicin liposomeloaded microbubbles can induce a 4-fold decrease of cell viability compared with treatment with free doxorubicin or doxorubicin liposome-loaded microbubbles alone. The therapeutic effectiveness is correlated to an ultrasound-triggered release of doxorubicin from the liposomes and an enhanced uptake of the free
doxorubicin by glioblastoma cells. The results obtained demonstrate that the combination of ultrasound and the doxorubicin liposome-loaded microbubbles can provide a new method of noninvasive image-guided drug delivery.
Investigators: J-M Escoffre, A. Novell, F. Remerand
Project leader: A. Bouakaz
Collaborations: M. Laffon (CHRU Tours, France), F. Tranquart (Bracco Research, Switzerland), M. Böhmer & C. Chlon (Philips Research Europe, Eindhoven, NL), I. Lentacker (Ghent University, Belgium), V. Gouilleux & T. Lecomte (CNRS UMR 7292).
Début de la page
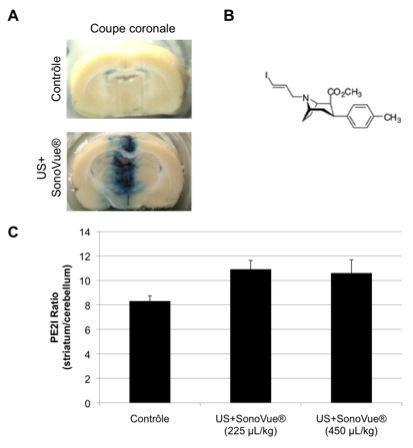 The blood-brain barrier (BBB) plays a major role in controlling the delivery of therapeutic and diagnostic agents to the brain. Advances in neuro-medical sciences have resulted in the development of new molecule delivery strategies that may be useful for therapy or diagnostic of many central nervous system diseases, but achieving a therapeutic effectiveness or a diagnostic imaging of these entities is often restricted by the BBB. The combination of focused ultrasound (FUS) and contrast agents has been shown to locally and noninvasively increase the permeability of the BBB. In this research project, we designed a sonoporation setup dedicated to BBB disruption and investigated the required ultrasound conditions to induce a transient increase of BBB permeability on mice, rats and lamps with and without craniectomy. We reported that the attenuation measurements of parietal bones for different animal and human skulls at the ultrasound frequency of 1 MHz. The prior knowledge of skull attenuation allows the optimization of the ultrasound amplitude. The results suggest that in-vivo experiments can be done without craniotomy in rodents by increasing the driving amplitude to compensate the attenuation. Preliminary in-vivo results show that a transient BBB disruption in rats.
The blood-brain barrier (BBB) plays a major role in controlling the delivery of therapeutic and diagnostic agents to the brain. Advances in neuro-medical sciences have resulted in the development of new molecule delivery strategies that may be useful for therapy or diagnostic of many central nervous system diseases, but achieving a therapeutic effectiveness or a diagnostic imaging of these entities is often restricted by the BBB. The combination of focused ultrasound (FUS) and contrast agents has been shown to locally and noninvasively increase the permeability of the BBB. In this research project, we designed a sonoporation setup dedicated to BBB disruption and investigated the required ultrasound conditions to induce a transient increase of BBB permeability on mice, rats and lamps with and without craniectomy. We reported that the attenuation measurements of parietal bones for different animal and human skulls at the ultrasound frequency of 1 MHz. The prior knowledge of skull attenuation allows the optimization of the ultrasound amplitude. The results suggest that in-vivo experiments can be done without craniotomy in rodents by increasing the driving amplitude to compensate the attenuation. Preliminary in-vivo results show that a transient BBB disruption in rats.Furthermore, sonoporation was successfully used to increase the specific fixation of a radiotracer in the striatum of treated rats. This innovative method provides a great potential for intracerebral delivery of molecular ligands that could be used for the therapy of brain diseases.
Investigators: J-M Escoffre, A. Novell
Project leader: A. Bouakaz
Collaborations: S. Chalon & S. Serrière (UMR 930, Tours), J-C Thiéry (INRA PRC, Nouzilly), F. Tranquart (Bracco Research, Switzerland).
Début de la page
